Patient participation in clinial trial design
SHARED DECISION MAKING – ROUND TABLE DISCUSSIONS
Introduction
During the 2nd ENMC General Assembly Meeting (GAM) held on 4–5 April 2025, we explored the topic of shared decision-making in clinical trial design. The session began with a presentation by Ingeborg Meijer, Chair of the ENMC, who explained that this discussion builds upon insights from the 235th ENMC workshop and the White Paper published in 2020. Due to time constraints, only one key theme from the White Paper was selected for discussion: clinical trial design.
The round table discussion centered around the following model:
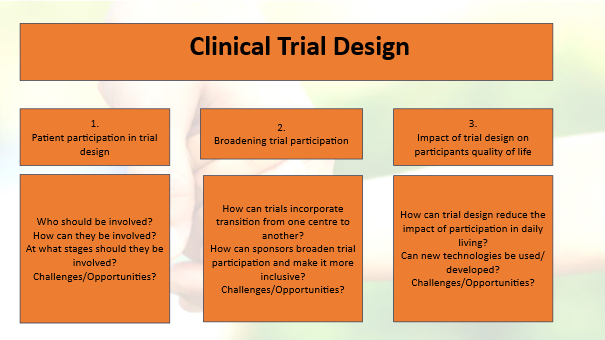
General summary
The discussions on patient participation were dynamic and insightful across all tables, reflecting the diverse expertise of stakeholders—including ENMC Associated Partners, members of the ENMC Company Forum, the Research Committee (RC), and the Executive Committee (EC).
Topic 1:
The overall consensus is that patients should be involved from the outset and throughout all stages of the process, using tailored approaches for specific aspects. Participation should include a diverse group of patients from various types of organizations. However, organizing such contributions in a systematic way is challenging. It requires careful planning, training, and time, while also managing expectations. When well-organized, patient involvement can enhance public trust and acceptance of both the initiation and outcomes of clinical trials.
Topic 2:
Broadening participation in clinical trials could help raise awareness and improve inclusion of underserved populations. This would require broad eligibility criteria to ensure real-world relevance of results, including considerations for health technology assessment (HTA) and quality of life from the outset. Therefore, comprehensive data collection and the use of validated endpoints should be implemented from the beginning. Such an approach would benefit from a network of trial centers operating within a hub-and-spoke model, ensuring no opportunities are missed and enabling remote assessments where appropriate. Transitioning patients between centers may lead to some loss in the consistency of outcome assessments; however, losing a patient entirely represents a far greater loss. This issue is particularly relevant during the transition from pediatric to adult care, such as when a child turns 18.
Suggestion: An ENMC workshop on clinical trials, with a particular focus on open-label extension (OLE) studies. What are the rationales for different approaches when children in trials turn 18? What practices are followed in different countries?
Topic 3:
Trial centers should be better equipped—both by providing adequate workspaces for staff and by supporting remote assessments and the use of wearables and other innovative technologies. These approaches should aim to deliver maximum value with minimal burden on patients and their families. They must also be paired with validated outcome measures and patient-reported outcome measures (PROMs). At the end of a trial, collecting patient feedback through a questionnaire on quality of life (QoL) impact could help improve future trial processes. Of course, bureaucracy and the regulatory approval of new tools and instruments remain ongoing challenges.
Conclusion
The roundtable discussions—marked by lively, in-depth, and diverse contributions—highlighted both the importance and the complexity of integrating shared decision-making into clinical trial design. As with any complex issue, there are no simple solutions or one-size-fits-all answers. Given the strong interest and valuable insights shared, this theme has significant potential to be developed into a full ENMC workshop to explore some of the key aspects raised. We invite interested participants to submit workshop applications.
