THEMED WORKSHOPS
In addition to the regular open workshop applications, where the topics are proposed by the workshop organisers, the ENMC also offers “Themed Workshops”.
This programme prepares the stepping stones for the future by listening to the needs of neuromuscular patients and related research. With a “Themed Call”, ENMC provides workshop funding opportunities for 1 or 2 Themed Workshops per year, focusing on two identified themes which:
- are of broader interest to more than one condition (transversal workshops)
- require a multidisciplinary approach
- are topics and/or diseases that are not often discussed in a workshop and are therefore unique
- are groundbreaking, strategic and require development of a sustainable platform in the future
At the 2nd ENMC General Assembly Meeting in April 2025, the ENMC Funding partners, Associated Partners, members of the Research Committee and members of the Company Forum selected two themes:
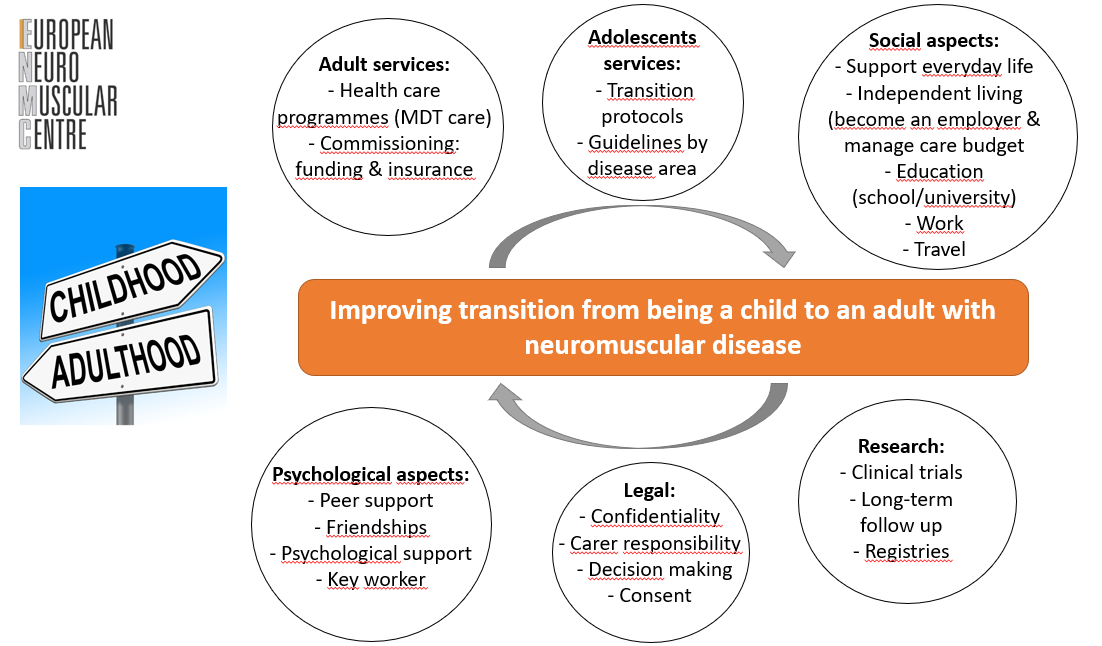
1. Improving transition from being a child to an adult with neuromuscular disease
2. Assessing meaningful long-term outcomes of gene therapy
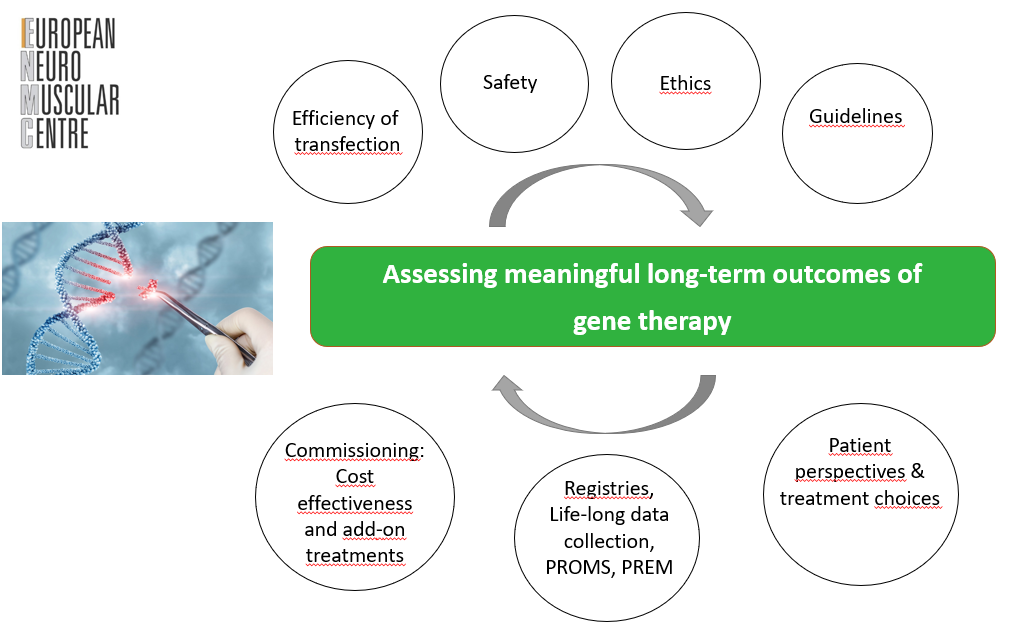
The themes are broad; therefore, the ENMC does not expect all sub-categories to be covered in a single workshop application. Organisers who wish to address one of these themes and apply for an ENMC workshop may focus on one or more topics related to the theme. Organisers should clearly define the rationale, background, objectives, and expected deliverables of the workshop, in accordance with the ENMC Workshop Application Guidelines and the additional Guidelines for Themed Workshop Applications.
The submission deadline is: 1 March 2026. If you seek support in this process, please do not hesitate to contact us and/or send a preliminary draft for a first check by the office: enmc@enmc.org.
